 - Download PDF-
- Download PDF-
SEEFOR 6 (1): 53-63
Article ID: 28
DOI: http://dx.doi.org/10.15177/seefor.14-18
Original scientific paper
Mycorrhizal Fungal Community of Poplars Growing on Pyrite Tailings Contaminated Site near the River Timok
Marina Katanić 1*, Saša Orlović 1, Tine Grebenc 2, Branislav Kovačević 1, Marko Kebert 1, Milan Matavulj 3, Hojka Kraigher 2
1 Institute of Lowland Forestry and Environment, University of Novi Sad, Antona Čehova 13, RS-21000 Novi Sad, Serbia
2 Slovenian Forestry Institute, Večna pot 2, SI-1000 Ljubljana, Slovenia
3 European University, Faculty of Pharmacy, Trg mladenaca 5, RS-21000 Novi Sad, Serbia
* Corresponding author: e-mail:
Citation:
KATANIĆ M, ORLOVIĆ S, GREBENC T, KOVAČEVIĆ B, KEBERT M, MATAVULJ M, KRAIGHER H 2015 Mycorrhizal Fungal Community of Poplars Growing on Pyrite Tailings Contaminated Site near the River Timok. South-east Eur for 6 (1): 53-63. DOI: http://dx.doi.org/10.15177/seefor.14-18
Received: 26 Sep 2014 / Accepted: 12 Dec 2014 / Published online: 29 Dec 2014
Cited by: CrossRef Google Scholar
Abstract
Background and Purpose: Mycorrhizal fungi are of high importance for functioning of forest ecosystems and they could be used as indicators of environmental stress. The aim of this research was to analyze ectomycorrhizal community structure and to determine root colonization rate with ectomycorrhizal, arbuscular mycorrhizal and endophytic fungi of poplars growing on pyrite tailings contaminated site near the river Timok (Eastern Serbia).
Materials and Methods: Identification of ectomycorrhizal types was performed by combining morphological and anatomical characterization of ectomycorrhizae with molecular identification approach, based on sequencing of the nuclear ITS rRNA region. Also, colonization of poplar roots with ectomycorrhizal, arbuscular mycorrhizal and dark septated endophytic fungi were analysed with intersection method.
Results and Conclusions: Physico-chemical analyses of soil from studied site showed unfavourable water properties of soil, relatively low pH and high content of heavy metals (copper and zinc). In investigated samples only four different ectomycorrhizal fungi were found. To the species level were identified Thelephora terrestris and Tomentella ellisi, while two types remained unidentified. Type Thelephora terrestris made up 89% of all ectomycorrhizal roots on studied site. Consequently total values of Species richness index and Shannon-Weaver diversity index were 0.80 and 0.43, respectively. No structures of arbuscular mycorrhizal fungi were recorded. Unfavourable environmental conditions prevailing on investigated site caused decrease of ectomycorrhizal types diversity. Our findings point out that mycorrhyzal fungal community could be used as an appropriate indicator of environmental changes.
Keywords: ectomycorrhiza, molecular identification, poplars, Timok, pyrite tailing
INTRODUCTION
Mining complex in the vicinity of Bor (Eastern Serbia) represents a considerable source of environmental pollution. Soil from a large area near the river Timok was contaminated by flotation tailing composed mainly of pyrite (FeS2). Consequently vegetation in this area suffered abiotic stress induced by a low pH, high content of copper and lead, deficiency of soil organic matter and severe deficiency of the available mineral nutrients [1].
Poplars are woody species suitable for phytoremediation purposes, because they can extract or incorporate into their aboveground tissues or stabilize in their root systems numerous contaminants from soil [2], [3].They are also well adapted to a broad range of climatic conditions and soils, have deep root systems, cycle large amounts of water and grow rapidly producing large amount of biomass [2], [3], [4].
Soil microorganisms such as mycorrhizal fungi could have important role in phytoremediation because they can modify bioavailability of heavy metals and/or increase plant growth [5]. Poplars can make functional associations with both ectomycorrhizal (ECM) and arbuscular mycorrhizal (AM) fungi [6].
Since these two mycorrhizal forms are known to prefer different climate and soil conditions such as nutrient content, pH and C/N ratio [7], dual colonization enables poplars to have broader ecological valence.
Mycorrhizal fungi facilitate the establishment and survival of vegetation under stress condition providing nutrients and water otherwise not accessible for plants [8]. With a net of their hyphae they can stabilize the tailing material and improve soil structure while with compounds produced by the extraradical mycelium can accumulate or chelate heavy metals [9]. In order to be efficient for use in phytoremediation techniques, mycorrhizal fungus has to satisfy two important conditions: tolerance to high concentration of heavy metals in soil and good functional compatibility with a plant used in phytoremediation [10][11].
Functional compatibility and stress tolerance in mycorrhiza are species specific and depend on both partner [11] therefore the information on the ECM community structure can provide valuable information about physiology of forest trees and functioning of forest ecosystems [12].
The aim of this research was to analyze ectomycorrhizal community structure and to determine root colonization rate with ectomycorrhizal, arbuscular mycorrhizal and endophytic fungi of poplars growing on pyrite tailings contaminated site near the river Timok. This information could be helpful in further research on creating inoculum for afforestation of sites contaminated with pyrite tailings.
MATERIAL AND METHODS
Physico-Chemical Properties of Soil from the Pyrite Tailings Contaminated Site Timok
Physical and chemical properties were determined in the surface layer of the soil (up to 30 cm). The following soil characteristics were analyzed: particle size distribution (%) by the international B-pipette method with the preparation in sodium pyrophosphate [13], determination of soil textural classes based on particle size distribution by using Atteberg classification, CaCO3 percentage (%) was measured volumetrically by using Scheibler’s calcimeter [14] and pH in H2O and KCl were determined by electrometric method with combined electrode on Radiometer pH meter.
Concentrations of heavy metals in soil were determined with Atomic Absorption Spectrophotometer (VARIAN AAS 240 FS). All analysis were performed in the laboratory of Soil Science in the Institute of Lowland Forestry and Environment in Novi Sad.
Site
Mycorrhizal fine roots were isolated from soil samples collected in the river land of the river Timok (N 44º00’29.96’’, E 22º21’54.48’’, 228 m a.s.l.) located about 20 km from Zaječar town, in Eastern Serbia. Site was covered with naturally grown cca 40 years old poplar trees (Populus alba L., P. nigra L., P. tremula L. and their hybrids) mixed with Amorpha fruticosa L., Betula pendula Roth and Alnus glutinosa L. Climate is temperate continental with the average annual precipitation in the area of 581.4 mm. Average temperature of the air in January is -0.2ºC, in July 22.4ºC, while the average yearly temperature is 11°C (The Republic Hydrometeorological Service of Serbia http://www.hidmet.gov.rs/).
Sampling
Five mature white poplar trees were randomly selected for sampling. For ECM community analysis two soil samples per tree were taken in July 2010, at a distance of about 1m from the tree trunk. A soil core of 274 ml volume and 18 cm deep was used for taking standardized samples [15]. In total, ten soil core samples were collected and kept stored at 4ºC for up to three months. Prior to mycorrhizal analysis each soil core was submerged in cold tap water to loosen the soil structure. Roots were carefully washed from soil and vital ECM root tips were separated from old, nonturgescent and nonmycorrhizal (ONN) root tips in water under a dissecting microscope. For the determination of root length colonization with ECM, AM and endophytic (END) fungi five additional soil samples per plant (in total 25) were taken in August 2011 using the same sampling approach.
Identification of Ectomycorrhizae
ECM types were identified by combining morphological and anatomical approach with molecular methods performed at the laboratory of the Department of Forest Physiology and Genetics in Slovenian Forestry Institute in Ljubljana, Slovenia.
Morphological and anatomical characteristics of each ECM type were assessed by a binocular Olympus SZX 12 (light source Olympus Highlight 3100, daylight filter) and microscope Olympus BX 51 (magnification 100-2000 x) following methodology proposed by Agerer [16] and Kraigher [17], and ECM descriptions published in Agerer [18], Agerer et al. [19], and Agerer and Rambold [20]. All fine root tips categories were manually counted under the stereomicroscope. Based on the presence and abundance of emanating elements, ECM types were also classified into the exploration types proposed by Agerer [21].
Molecular identification was based on nucleotide sequencing of ITS (Internal Transcribed Spacer) regions in nuclear ribosomal DNA. This molecular marker is considered as the best for fungi identification [22]. After DNA extraction from 5-20 root tips with a PlantDNeasy Mini Kit (Qiagen, Hilden, Germany) from each ECM type ITS region was amplified with ITS 1f and ITS 4 primer pair [23]. DNA fragments were separated in and excised from agarose gel and purified with Wizard® SV Gel and PCR Clean-up System (Promega Corporation, Madison, WI, USA). Sequencing was performed commercially at Macrogen Inc. (Seoul, Rep. of Korea). Species, genus or family of ectomycorrhizal fungi were determined by comparing sequences to the ones deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/index.html) and Unite databases [24].
Root Colonization
Before evaluation, poplar’s fine roots from 5 soil samples were isolated from soil, separated from the roots of herbaceous species by means of visual inspection and jointed together in one sample. Extracted roots were gently washed and cleared in 10% potassium hydroxide and stained with Trypan blue in lactoglycerol according to Kormanik and McGraw [25] and Karlin´ski et al. [26]. Colonization of poplar roots by ECM, AM and END fungi was evaluated using the intersection method by McGonigle et al. [27] modified by Karlin´ski et al. [26] at 200× magnification. A minimum of 200 line intersections per subsample (microscopic slide) were scored for the presence of AM structures (hyphae, vesicles, arbuscules, and coils), ECM or END fungi. Cross section without fungal structures was counted as “empty root”. The results are presented as a percentage of root length colonized i.e. partition of number of particular fungal structures in total number of cross sections.
Data Analysis
Diversity indices (Shannon-Weaver index, Species richness index, Evenness, Equitability and Berger-Parker index) were calculated per sample and per site in the way that ECM community data were pooled after formulas given by Atlas and Bartha [28]:
- Species richness (d): d = (S-1) / log(10)N,
where S - number of ECM types, N - number of all mycorrhizal tips;
- Shannon Weaver diversity index (H): H = C/N (N∙logN - ∑ni∙log ni),
where C - 2,3, N - number of all mycorrhizal tips, ni - number of mycorrhizal tips of individual ECM type;
- Evenness (e): e = H / logS,
where H - Shannon Weaver diversity index, S - number of ECM types;
- Equitability (J): J = H / Hmax,
where H - Shannon Weaver diversity index, Hmax - theretical maximal H assuming each ECM type was represented with one mycorrhizal tip;
- Berger-Parker evenness index (BP): BP = 1 - (Nmax / N),
where Nmax = number of mycorrhizal tips of the most frequent ECM type, N=number of all mycorrhizal tips.
Relative abundance of ECM types was calculated as a ratio between the tips number of individual ECM type and total number of ECM tips.
RESULTS
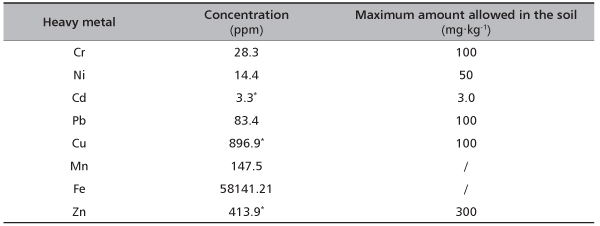
Physical and chemical analysis of examined soil samples from pyrite tailings contaminated site showed high content of total sand (91.4%), moderately acid pH (4.91) (according to Dugalić and Galić [29]) and very low concentration of nitrogen (0.06%) (Table 1). According to its granulometric content soil can be classified in the sand texture class. Also, it belongs to the group of technogenous soils, type deposol [30]. Origin of this soil type is related to the undeveloped alluvial soil (fluvisol) but subsequently influenced by the spilling of pyrite tailings and Fe2S deposition causing lower pH and contamination with heavy metals. Comparison of heavy metals content in soil from studied site with the National legislation limits [31] has shown that concentration of copper was almost 9x higher than its maximum allowed amount, while zinc was increased and cadmium was slightly above the allowed amount (Table 2).
TABLE 1. Granulometric composition and some chemical properties of soil from Timok site

TABLE 2. Concentrations of heavy metals in soil from Timok site (with their maximum amounts allowed in the soil according to the National legislation)
Analyzing 34042 fine roots on Timok site four ECM types were recorded, while values of Species richness index and Shannon-Weaver index were 0.80 and 0.43, respectively. In average 1.4 ECM types, 550.6 vital mycorrhizal root tips and 3404 all fine roots were recorded per soil sample. Consequently, average values of diversity indices were extremely low (Table 3).
TABLE 3. Total values and average values per sample (±standard error) for number of ectomycorrhizal (ECM) types, number of vital ECM roots, old, nonturgescent and nonmycorrhizal roots, number of all roots, percentage of vital mycorrhizal roots and diversity indices on investigated site Timok (based on 10 samples)
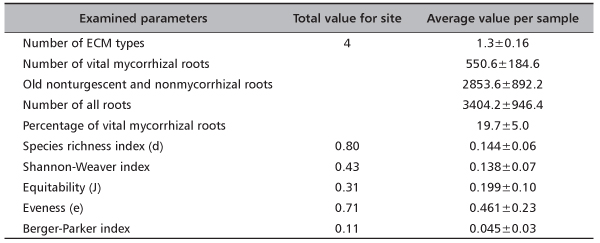
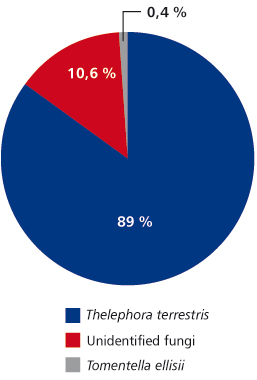
FIGURE 1. Ectomycorrhizal fungi community structure of poplars from Timok site
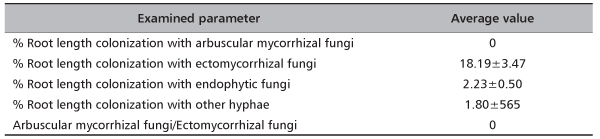
In total, at site Timok four ECM types were recorded. Two of them were identified to the species level: Thelephora terrestris and Tomentella ellisii, while two ECM types remained unidentified (Table 4). Thelephora terrestris made up 89% of all ECM roots (Figure 1) and consequently medium distance exploration type made up almost 90% of all ECM roots on this site (data were not shown). Although both fungal groups, Ascomycota and Basidiomycota had two members, Basidiomycota was much more abundant and made up 89.4% of all ECM roots (data not shown). In examined poplar roots no AM fungal structures were observed, while ECM and END fungi colonized 18.19% and 2.23% of root length, respectively (Table 5).
TABLE 4. Identified ectomycorrhizal fungi on the basis on the similarities with sequences given in the internet basis GenBank and UNITE and phylogenetic analyses
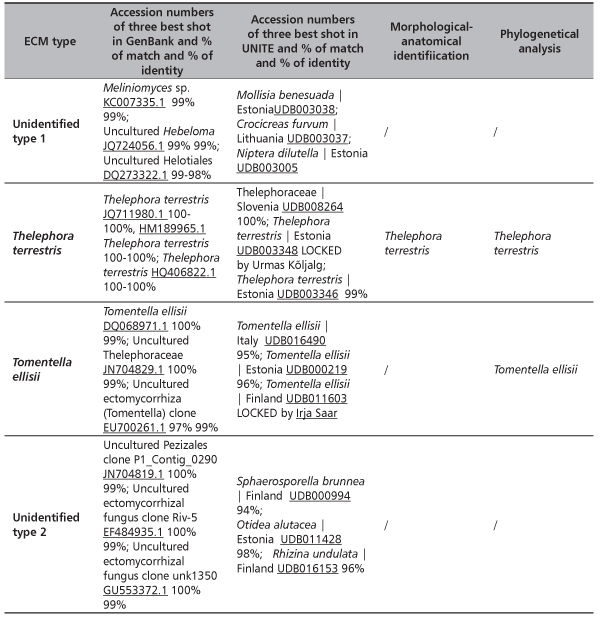
TABLE 5. Average values (±standard error) of poplar roots colonization with ectomycorrhizal, arbuscular mycorrhizal and dark septated endophytic fungi at Timok site
DISCUSSION
The main causes of extreme conditions at the pyrite tailings contaminated site near the river Timok were unfavourable water-air properties of analyzed soil, low pH and contamination with heavy metals copper and zinc. In such soils with high proportion of total sand, content of water available to the plants is low. Also, moderately acidic pH of the soil could not be favourable for while poplars that are known to prefer fluvisol soil type with slightly or moderately alkaline pH [32].
Analysis of fine roots’ number, values of diversity indices and relative abundances of fungi that form ECM association with poplars from site Timok enabled comparison of ECM fungal community from studied site with ones from other similar sites.
On site Timok average number of fine roots per 1 dm3 of soil was 12425.3/dm3, while number of vital ECM roots was 2009.7/dm3. Recorded values were much lower in comparison with results of Krpata et al. [33] who counted 1735-4263 mycorrhizal root tips in 100 ml of soil in aspen stand from site contaminated with heavy metals. On the other hand, in the experimental field with increased ozone concentration in the air, average value of fine poplar roots was 2599.1/dm3 in control treated with water and 4573.5/dm3 in the antiozonant protected plants [34].
Comparison of Shannon-Weaver diversity index recorded on site Timok (0.43) with its common values, which are in the range 1.5-3.5 according to Urbančič and Kutnar [35], showed that diversity on studied site was decreased. Low diversity of ECM types supports the observation that pollution could cause disappearance of sensitive ECM fungi and increase abundance of tolerant ones, decreasing in that way its diversity [36]. High proportion of sand in the soil and low content of water available to the plants (data not shown) suggested unfavourable conditions for development of mycorrhizal community. In addition, adverse influence of drought conditions on mycorrhizal fungi was proven by Lodge [37].
Under extreme abiotic conditions on site Timok only four ECM types were recorded. Similar results were obtained in studies of ECM communities on the sites under the influence of stress factors. On the site contaminated with heavy metals, Regvar et al. [38] recorded 7 ECM types on birch. On the clone Populus nigra × maximowiczii cv. Max grown as short rotation crop, Hrynkiewicz et al. [39] found 5 ECM types. On the other hand, Krpata et al. [33] recorded 54 ECM types on aspen from the site contaminated with heavy metals. On two sites polluted with zinc, Mleczko [40] recorded 23 ECM types, and the same number of ECM types was observed on uranium polluted site [42].
In our work data were collected in summer 2010 and 4 ECM types (Thelephora terrestris, Tomentella ellisii and two unidentified types) were recorded. On the same site in winter 2009, Katanić et al. [41] recorded 6 ECM types: Tricholoma scalpturatum (Fr.) Quel., Tuber puberulum Berk. & Br., Thelephora sp., Helotiales sp., Sebacinales sp. and Sordariomycetidae sp. Seasonal change in ECM community supports hypothesis of Koide et al. [43] that temporal distribution within ECM fungal community reduces competition among species.
Tomenteloid fungi belong to the most frequent and most abundant ECM partners of coniferous and broadleaved trees in woods of Europe and North America [44]. Kraigher and Al Sayegh Petkovšek [45] noted that, beside Cenococcum geophilum, fungus Thelephora terrestris is one of the rare fungi which form developed ECM in drought conditions. In addition, teleforoid fungi could play crucial role in forest ecosystems under the influence of stress [45]. Results from Timok are in accordance with their studies. Dominance of medium distance exploration type reveiled in our study is concordant with results of Rudawska et al. [46] who recorded high proportion of this exploration type on the locality contaminated with heavy metals.
High intraspecies variability could be found in ECM fungi according to Cairney [47], while Johnson et al. [48] consider that groups of genotypes can affect ecosystem processes in the same way as species do. Within species, individuals differ a great deal in important reproductive and functional characters. Since, ECM fungus Thelephora terrestris is adapted to extreme conditions on the site Timok it could be assumed that strain from Timok developed some physiological adaptations to the extreme environmental factors.
In preparation of mycorrhizal inoculum for afforestration of demaged or contaminated sites, autochthonous strains of fungi, well adapted to such envionmental conditions, should be chosen [17], [49]. Strain of ECM fungus Thelephora terrestris from Timok could be proposed as a basis for creating inoculum for afforestation of this one or similar localities with extreme conditions.
CONCLUSIONS
According to the presented results it could be concluded that physico-chemical soil properties of studied site were unfavourable considering poor water properties, relatively low pH and high content of heavy metals (copper and zinc). Only four different ectomycorrhizal fungi were found from which fungus Thelephora terrestris made up 89% of all ectomycorrhizal roots. Total values of Species richness index and Shannon-Weaver diversity index were 0.80 and 0.43, respectively. No structures of arbuscular mycorrhizal fungi were recorded. The presented results suggest that described environmental conditions on investigated site caused decrease of ectomycorrhizal types diversity. Our findings point out that mycorrhyzal fungal community could be used as an appropriate indicator of environmental changes.
Acknowledgements
The study was realized by project III43007 “Studying climate change and its influence on the environment: impacts, adaptation and mitigation” financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia and co-financed by the Slovenian Research Agency through Research Programme P4-0107 “Forest Biology, Ecology and Technology”, through the Scholarship Ad futura (OMEGA D.O.O., for MK)
REFERENCES
- ANTONIJEVIĆ MM, MARIĆ M 2008 Determination of the content of heavy metals in pyrite contaminated soil and plants. Sensors 8 (9): 5857-5865. DOI: http://dx.doi.org/10.3390/s8095857
- DI BACCIO D, TOGNETTI R, SEBASTIANI L, VITAGLIANO C 2003 Responses of Populus deltoides × Populus nigra (Populus × euramericana) clone I-214 to high zinc concentrations. New Phytol 159 (2): 443-452. DOI: http://dx.doi.org/10.1046/j.1469-8137.2003.00818.x
- PILIPOVIĆ A, NIKOLIĆ N, ORLOVIĆ S, PETROVIĆ N, KRSTIĆ B 2005 Cadmium phytoextraction potential of poplar clones (Populus spp.). Z Naturforsch C 60 (3-4): 247-251
- NEWMAN LA, STRAND SE, CHOE N, DUFFY J, EKUAN G, RUSZAJ M, SHURLEFF BB, WILMOTH J, et al. 1997 Uptake and Biotransformation of Trichloroethylene by Hybrid Poplars. Environ Sci Technol 31 (4): 1062-1067. DOI:http://dx.doi.org/1021/es960564w
- CICATELLI A, LINGUA G, TODESCHINI V, BIONDI S, TORRIGIANI P, CASTIGLIONE S 2010 Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot-London 106 (5): 791-802. DOI: http://dx.doi.org/10.1093/aob/mcq170
- MOLINA R, MASSICOTTE H, TRAPPE JM 1992 Specificity phenomena in mycorrhizal symbiosys: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman & Hall, New York, NY, USA, pp 357-423
- READ DJ 1991 Mycorrhizas in Ecosystems. Experientia 47 (4): 376–391. DOI: http://dx.doi.org/10.1007/BF01972080
- SMITH SE, READ DJ 2008 Mycorrhizal symbiosis. Academic Press - Elsevier, Cambridge, UK, 787 p
- TURNAU K, ORLOWSKA E, RYSZKA P, ZUBEK S, ANIELSKA T, GAWRONSKI S, JURKIEWICZ A 2006 Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in southern Poland. In: Twardowska I, Herbert EA, Häggblom MM, Stefaniak S (eds) Soil and Water Pollution Monitoring, Protection and Remediation, Proceedings of the NATO Advanced Research Workshop on Viable Methods of Soil and Water Pollution Monitoring, Protection and Remediation, Krakow, Poland, 27 June-1 July 2005. Springer, Dordrecht, The Netherlands, pp 533-531. DOI: http://dx.doi.org/10.1007/978-1-4020-4728-2_35
- KHAN AG., KUEK C, CHAUDHRY TM, KHOO CS, HAYES WJ 2000 Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41 (1-2): 197-207. DOI: http://dx.doi.org/10.1016/S0045-6535(99)00412-9
- GIANINAZZI-PEARSON V 1984 Host-fungus specificity, recognition and compatibility in mycorrhizae. In: Verma DPS, Hohn T (eds) Genes involved in microbe-plant interactions. Plant Gene Research, Springer-Verlag, Wien, pp 225-253. DOI: http://dx.doi.org/1007/978-3-7091-8739-5_8
- KRAIGHER H, AL SAYEGH-PETKOVŠEK S, GREBENC T, SIMONČIČ P 2007 Types of ectomycorrhiza as pollution stress indicators: case studies in Slovenia. Environ Monit Assess 128 (1): 31-45. DOI: http://dx.doi.org/10.1007/s10661-006-9413-4
- BOŠNJAK Đ, HADŽIĆ V, BABOVIĆ D, KOSTIĆ N, BURLICA Ĉ, ĐOROVIĆ M, PEJKOVIĆ M, MIHAJLOVIĆ TD, et al. 1997 Methods of investigation and determination of soil characteristics (in Serbian). Yugoslavian society for soil investigation, Commission for soil physics, Novi Sad, Serbia, 278 p
- HADŽIĆ V, BELIĆ M, NEŠIĆ LJ 2004 Pedology practicum (in Serbian). Faculty of Agriculture, Novi Sad, Serbia, 80 p
- KRAIGHER H 1999 Diversity of types of ectomycorrhizae on Norway spruce in Slovenia. Phyton 39 (3): 199-202
- AGERER R 1991 Characterization of ectomycorrhiza. In: Norris JR, Read DJ, Varma AK (eds) Techniques for the study of mycorrhiza .Methods in Microbiology 23, Academic Press, London, UK, pp 25-72
- KRAIGHER H 1996 Types of mycorrhiza: taxonomy, importance, application (in Slovenian). Zbornik gozdarstva in lesarstva 49: 33-66
- AGERER R 1987-2008 Colour Atlas of Ectomycorrhizae 1st-13th Einhorn-Verlag, Schwäbisch Gmünd, Germany
- AGERER R, DANIELSON R M, EGLI S, INGLEBY K, LUOMA D, TREU R 2001-2006 Descriptions of ectomycorrhizae 1st–10th Einhorn-Verlag, Schwäbisch Gmünd, Germany
- AGERER R, RAMBOLD G 2004-2014 DEEMY – An Information System for Characterization and Determination of Ectomycorrhizae. URL: http://www.deemy.de (15 November 2014)
- AGERER R 2001 Exploration types of ectomycorrhizae. Mycorrhiza 11 (2): 107-114. DOI: http://dx.doi.org/10.1007/s005720100108
- KÕLJALG U, NILSSON RH, ABARENKOV K, TEDERSOO L, TAYLOR AFS, BAHRAM M, BATES ST, BRUNS TD, et al. 2013 Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22 (21): 5271-5277. DOI: http://dx.doi.org/10.1111/mec.12481
- GARDES M, BRUNS TD 1993 ITS primers with enhanced specificity for basidiomycetes- application to the identification of ectomycorrhizae and rusts. Mol ecol 2 (2): 113-118. DOI: http://dx.doi.org/10.1111/j.1365-294X.1993.tb00005.x
- ABARENKOV K, NILSSON RH, LARSSON K-H, ALEXANDER IJ, EBERHARDT U, ERLAND S, HØILAND K, KJØLLER R, et al. 2010 The UNITE database for molecular identification of fungi - recent updates and future perspectives. New Phytol 186 (2): 281-285. DOI: http://dx.doi.org/10.1111/j.1469-8137.2009.03160.x
- KORMANIK PP, MCGRAW AC 1982 Quantification of vesicular – arbuscular mycorrhizae in plant roots. In: Schenck NC (ed) Methods and principles of mycorrhizal research. American Phytopathological Society, St. Paul, MN, USA, pp 37-45
- KARLIŃSKI L, RUDAWSKA M, KIELISZEWSKA-ROKICKA B, LESKI T 2010 Relationship between genotype and soil environment during colonization of poplar roots by mycorrhizal and endophytic fungi. Mycorrhiza 20 (5): 315-324. DOI: http://dx.doi.org/10.1007/s00572-009-0284-8
- MCGONIGLE TP, MILLER MH, EVANS DG, FAIRCHILD GL, SWAN JA 1990 A new method, which gives an objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115 (3): 495–501. DOI: http://dx.doi.org/10.1111/j.1469-8137.1990.tb00476.x
- ATLAS R, BARTHA R 1981 Introduction to microbiology. Addison-Wesley, Reading, UK, pp 242-244
- DUGALIĆ G, GAJIĆ B 2005 Pedology practicum (in Serbian). Agronomy faculty, Čačak, Serbia, pp 147-152
- ŠKORIĆ A, FILIPOVSKI G, ĆIRIĆ M 1985 Classification of Yugoslav Soils (in Bosnian), Academy of Sciences and Arts of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina
- THE MINISTRY OF AGRICULTURE, FORESTRY AND WATER MANAGEMENT IN AGREEMENT WITH THE MINISTRY OF ENVIRONMENTAL PROTECTION 1994 Regulations about allowed quantities of dangerous and harmful matters in soil and irrigating waters and methods about their analysis (in Serbian), Official Gazette 23/94, Belgrade, Serbia
- PEKEČ S 2010 Pedological and hydrological characteristics of protected part of alluvial plain in Middle Danube (in Serbian with English summary). PhD thesis, University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia, 221 p
- KRPATA D, PEINTNER U, LANGER I, WALTER JF, SCHWEIGER P 2008 Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol res 112 (9): 1069-1079. DOI: http://dx.doi.org/10.1016/j.mycres.2008.02.004
- KATANIĆ М, PAOLETTI Е, ORLOVIĆ S, GREBENC T, KRAIGHER H 2014 Mycorrhizal status of an ozone-sensitive poplar clone treated with the antiozonant ethylene diurea. Eur J For Res 133 (4): 735-743. DOI: http://dx.doi.org/1007/s10342-013-0751-9
- URBANČIČ M, KUTNAR L 1998 The variety of soil conditions and ground vegetation of forests in moraine of Pokljuka plateau (in Slovenian with English summary). In: Diaci J (ed) Mountain forest: conference proceedings, the 19th Forestry Days, Logar Valley, Slovenia, 26-27 March 1998. Biotechnical Faculty of University Ljubljana, Ljubljana, Slovenia, pp 223-241
- AL SAYEGH-PETKOVŠEK S, POKORNY B 2006 Fungi as responsive and accumulative bioindicators of forest site pollution in the Šalek valley (in Slovenian with English summary). Zbornik gozdarstva in lesarstva 81: 61-71
- LODGE DJ 1989 The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix. Plant Soil 117 (2): 243-253. DOI: http://dx.doi.org/10.1007/BF02220718
- REGVAR M, LIKAR M, PILTAVER A, KUGONIČ N, SMITH JE 2010 Fungal community structure under goat willows (Salix caprea) growing at metal polluted site: the potential of screening in a model phytostabilisation study. Plant Soil 330 (1-2): 345-356. DOI: http://dx.doi.org/10.1007/s11104-009-0207-7
- HRYNKIEWICZ K, BAUM C, LEINWEBER P, WEIH M, DIMITRIOU I 2010 The significance of rotation periods for mycorrhiza formation in short rotation coppice. For Ecol Manage 260 (11): 1943-1949. DOI: http://dx.doi.org/10.1016/j.foreco.2010.08.020
- MLECZKO P 2004 Mycorrhizal and saprobic macrofungi of two zinc wastes in southern Poland. Acta Biol Cracov Bot 46: 25-38
- KATANIĆ M, ORLOVIĆ S, GREBENC T, BAJC M, GALIĆ Z, KEBERT M, KRAIGHER H 2011 Mycorrhizal fungi on poplars from a pyrite contaminated site. In: Orlović S (ed) STREPOW: Workshop Proceedings, Andrevlje-Novi Sad, Serbia, 23-24 February 2011. Institute of Lowland Forestry and Environment, Novi Sad, Serbia, pp 305-312
- STAUDENRAUSCH S, KALDORF M, RENKER C, LUIS P, BUSCOT F 2005 Diversity of the ectomycorrhiza community at an uranium mining heap. Biol Fert Soils 41 (6): 439-446. DOI: http://dx.doi.org/10.1007/s00374-005-0849-4
- KOIDE R T, SHUMWAY DL, XU B, SHARDA JN 2007 On temporal partitioning of a community of ectomycorrhizal fungi. New Phytol 174 (2): 420-429. DOI: http://dx.doi.org/10.1111/j.1469-8137.2007.02000.x
- DAHLBERG A 2001 Community ecology of ectomycorrhizal fungi: an advancing interdisciplinary field. New Phytol 150 3: 555-562. DOI: http://dx.doi.org/10.1046/j.1469-8137.2001.00142.x
- KRAIGHER H, AL SAYEGH-PETKOVŠEK S 2011 Mycobioindication of stress in forest ecosystems. In: Rai M, Varma A (eds) Diversity and biotechnology of ectomycorrhizae. Soil biology 25, Springer-Verlag Berlin Heidelberg, Germany, pp 301-322
- RUDAWSKA M, LESKI T, STASIŃSKA M. 2011 Species and functional diversity of ectomycorrhizal fungal communities on Scots pine (Pinus sylvestris) trees on three different sites. Ann For Sci 68 (1): 5-15. DOI: http://dx.doi.org/10.1007/s13595-010-0002-x
- CAIRNEY JWG 1999 Intraspecific physiological variation: implications for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9 (3): 125-135. DOI: http://dx.doi.org/1007/s005720050297
- JOHNSON D, IJDO M, GENNEY DR, ANDERSON IC, ALEXANDER IJ 2005 How do plants regulate the function, community structure and diversity of mycorrhizal fungi? J Exp Bot 56 (417): 1751–1760. DOI: http://dx.doi.org/10.1093/jxb/eri192
- DODD JC, THOMSON BD 1994 The screening and selection of inoculants arbuscular and ectomycorrhizal fungi. Plant Soil 159 (1): 149-158. DOI: http://dx.doi.org/10.1007/BF00000104
© 2015 by the Croatian Forest Research Institute. This is an Open Access paper distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0).

